A successful system changeover makes the difference between on-time production and complete halts.
Recently, our Customer Success team visited a site that produces 150+ supplement products on three assembly lines. This seems overwhelming, but most plants cannot dedicate a single system to every item they sell.
This challenge spans industries from supplements to apparel and beyond. Consider a clothing brand that uses the same line to produce synthetic and cotton garments. Even without oversight from regulatory bodies, factors such as fiber contamination and incorrect labeling can severely damage brand integrity.
How do big-name manufacturers ensure quality when their machines are calibrated for multiple products and specifications? Through a detailed system changeover process.
Daily changeover activities aren't foolproof on their own. Compliance is at risk every time, especially for industries adhering to strict FDA manufacturing regulations. Procedures must be thoroughly documented and QA'd for efficacy.
Instead of building changeovers on gut feelings, a CMMS can help maintenance managers plan changeovers around specific equipment needs and regulations to stay GMP compliant.
Why Changeover Times Matter
A system changeover is the process of transitioning assembly line equipment from producing one product to another.
Using our supplement customer as an example, let's say they use the same line to produce a powdered multivitamin on Monday and a protein blend on Tuesday. Before firing up the machines on Tuesday, the team must perform full line clearance.
Think of it like washing a dirty plate before eating again. The technician will clean any remaining residue and contact surfaces to prevent cross-contamination and verify that no allergens are present from the previous batch. Aspects like fill weights and packaging are also reconfigured here, preserving product integrity and consistency.
Changeover processes are essential for consumer safety, but they also take effort and maintenance resources. In a competitive manufacturing landscape where speed-to-market is everything, even small delays can lead to missed deadlines and slower sales.
In a 202 case study of a major European food manufacturer, the company was experiencing significant losses due to long, inefficient changeover times. Their machine operators mainly relied on memory for protocols, causing different steps to be taken between shifts. The outcome: unexpected downtime and wasted materials.
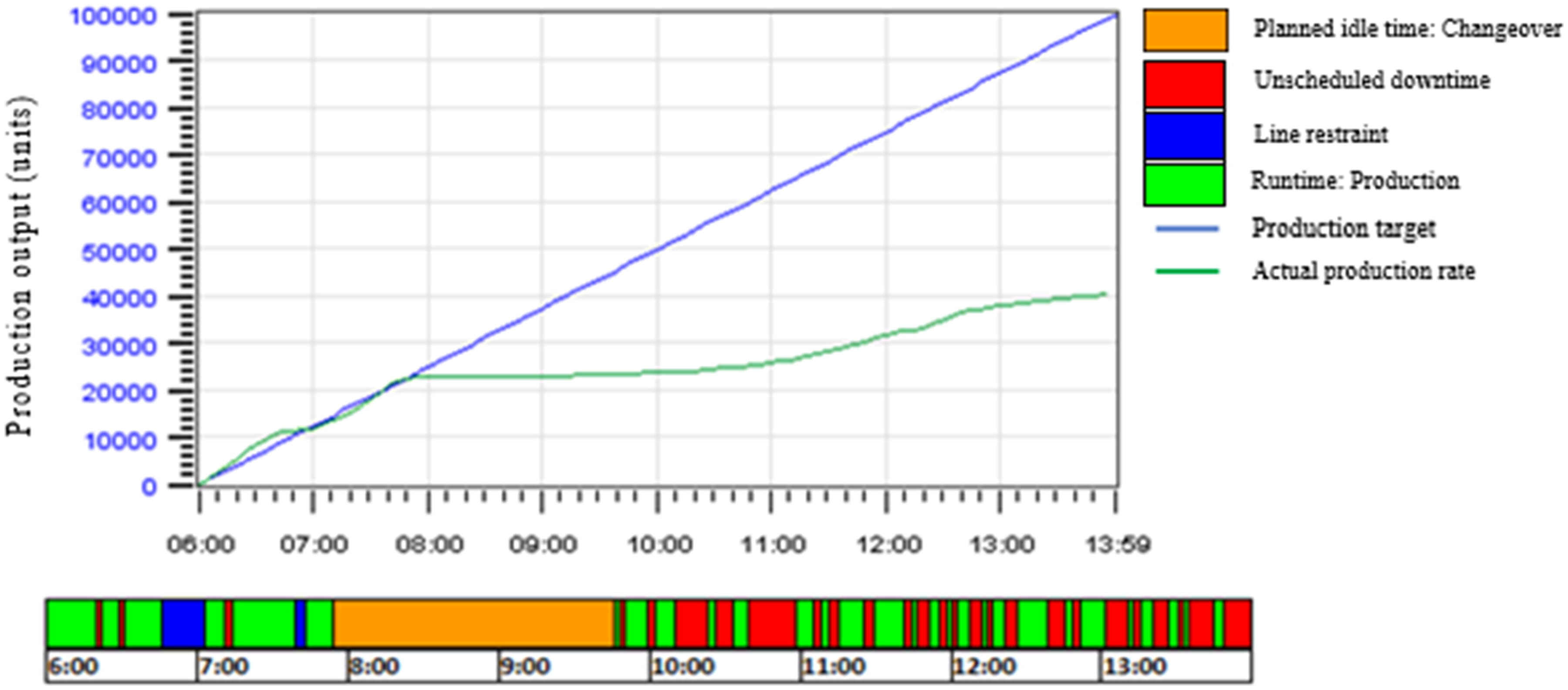
Their downfall wasn't the lack of a changeover process; it was documentation. When structured tasks were mapped out in their CMMS, the company could standardize instructions while identifying activities that didn't add value.
These changes solidified measurable improvements beyond their problem areas. On top of reducing changeover times and food waste, scheduling accuracy and line utilization also saw a boost from CMMS-driven communication.
How a CMMS Fills System Changeover Gaps
Proper sanitization is crucial for any machine producing consumable goods. However, changeovers are more complex when part replacements and reconfigurations are involved.
A CMMS streamlines necessary activities in a guide for faster, easier execution.
Let's revisit our supplement customer. If this company produces fish oil tablets on Wednesday and a vegan collagen powder on Thursday, extreme caution must be exercised to prevent cross-contamination and meet sustainability claims.
Here's where MVP One becomes a strategic advantage in system changeover compliance.
- Equipment Health Baseline: MVP One tracks asset performance and flags wear-and-tear trends. This indicates which machines are due for service before it impacts changeover quality.
- Cleaning and Replacement Schedules: Preventive maintenance schedules are built into the software, ensuring cleaning and part swaps happen consistently. Seamlessly identify what steps to take when switching between materials with different compliance requirements.
- PM Automation with Checklists and Instructions: Technicians receive automated work orders triggered by tailored alerts. The instructions and compliance prompts in each work order template reduce human error and expedite execution.
- Real-Time Communication: Every action taken during a system changeover is logged instantly through features like statuses and Huddle. Enhance cross-team visibility with digital sign-offs before the lines start running again.
- Audit Histories and Compliance Adherence: MVP One maintains a full audit trail, demonstrating ongoing adherence to internal quality standards, external FDA manufacturing regulations, and sustainability certifications.
Creating a System Changeover Process
A detailed system changeover process doesn't just protect brands. It's designed to keep teams on the same page for equipment upkeep as product demand soars.
With a CMMS like MVP One, plants can retrofit their changeover PMs to match labor and inventory availability. This ensures operations run at maximum capacity with minimal waste.
If a plant aims to uphold compliance within lean resources, the flexible planning and scheduling workflows in MVP One are the perfect starting points.
MVP One's Ultimate Changeover Checklist
- Preparation
- Review Work Order History: Our work module saves every work order created for an asset. Maintenance leads and technicians can assess this information to create a baseline for equipment health and strategize changeover job plans.
- Generate Changeover Work Orders: Create a daily, weekly, or monthly PM depending on product rotation frequency, then set due dates to alert teams of upcoming downtime.
- Create Role-Based Alerts: Attach People IDs to ensure someone with the right skills is available for changeover repairs. These technicians receive custom alerts when they're needed to perform a changeover PM.
- Verify Part and Inventory Availability: Add part and inventory IDs in automated PMs to check availability before working. Technicians can also see if necessary parts are running low to facilitate reorders and reduce part-related downtime.
- Line Clearance and Cleaning
- Work Instructions for Line Clearance: Maintenance planners can add step-by-step cleaning and replacement instructions to each work order. Photos and manufacturer manuals can also be attached for further clarification.
- Removing Product-Specific Materials: To begin line clearance, all product-specific materials need to be cleared, such as labels, packaging, and ingredients unique to the previous product. Follow work order instructions to verify the correct removal steps have been taken.
- Changeover Cleaning Procedure: Once product-specific materials are removed, the full line can be cleaned and sanitized. With MVP One's changeover work orders, cleaning materials can be added to each piece of equipment in a system for easier tracking.
- Logging Completion: Cleaning and replacement steps can be added as checkboxes to instructions and marked complete as the technician works.
- Cross-Contamination Prevention and Allergen Control
- Residue Inspection: Before reconfiguring equipment specs for the next product, technicians should inspect all contact surfaces for leftover residue. MVP One allows maintenance planners to include inspection checkpoints throughout instructions to evaluate cleaning quality.
- Documenting Control Measures: Any allergen control or sanitation protocols (such as separate cleaning materials or tools) should be documented in system changeover work orders. Our system provides attachment options to log these measures for future audits.
- Visual Inspection and Swab Test: Technicians should perform visual inspections and swab tests to verify full residue removal. Results can be recorded directly in our CMMS as an attached test report, supporting full day-to-day compliance with FDA manufacturing regulations.
- Equipment Reconfiguration
- Adjust Machine Settings: Once inspections are cleared, machine parameters can be updated to match the next product's requirements. Document the asset's settings in the work order's configuration notes for traceability.
- Replace or Recalibrate Tooling: Swap out product-specific tools like nozzles, molds, blades, seals, and more, then recalibrate sensors or controls. Add these tasks to PMs with part IDs and calibration instructions to ensure assembly consistency.
- Update Configuration Records: After completing reconfigurations, technicians can log adjustments in MVP One to ensure future system changeovers are based on accurate, up-to-date settings.
- Compliance Documentation
- Attach GMP/FDA Documentation: Include any relevant regulatory documents (SOPs, cleaning logs, validation forms, etc.) as an attachment. These guidelines can be added to any work order, making it easy to centralize compliance records for every system.
- Technician Sign-Offs: Require digital sign-offs from technicians and maintenance managers to confirm each step was completed according to protocol. Our mobile interface enables real-time sign-offs to track changeover progress instantly between sites.
- Log Audit Trail: Every action taken over changeover is automatically logged into MVP One, creating an airtight audit trail. This supports internal reviews and external inspections, solidifying complete transparency.
- Final QA
- Run First Article Inspection: Before full production resumes, run a first article to confirm inspections and recalibrations were done correctly. Log results in the changeover work order and link them to any QA documentation.
- Verify Labeling and Packaging: Double-check that all labeling and packaging components match the new product specs. Include visual references and checklist items to confirm accuracy.
- Close Work Order: Once all tasks are complete, the technician and manager can close the work order. After the work order is closed, MVP One automatically updates the asset's maintenance history.
- Follow-Up Reporting: Managers can generate changeover activity summaries at whatever cadence they prefer to identify labor usage, part check-out, or compliance improvements.
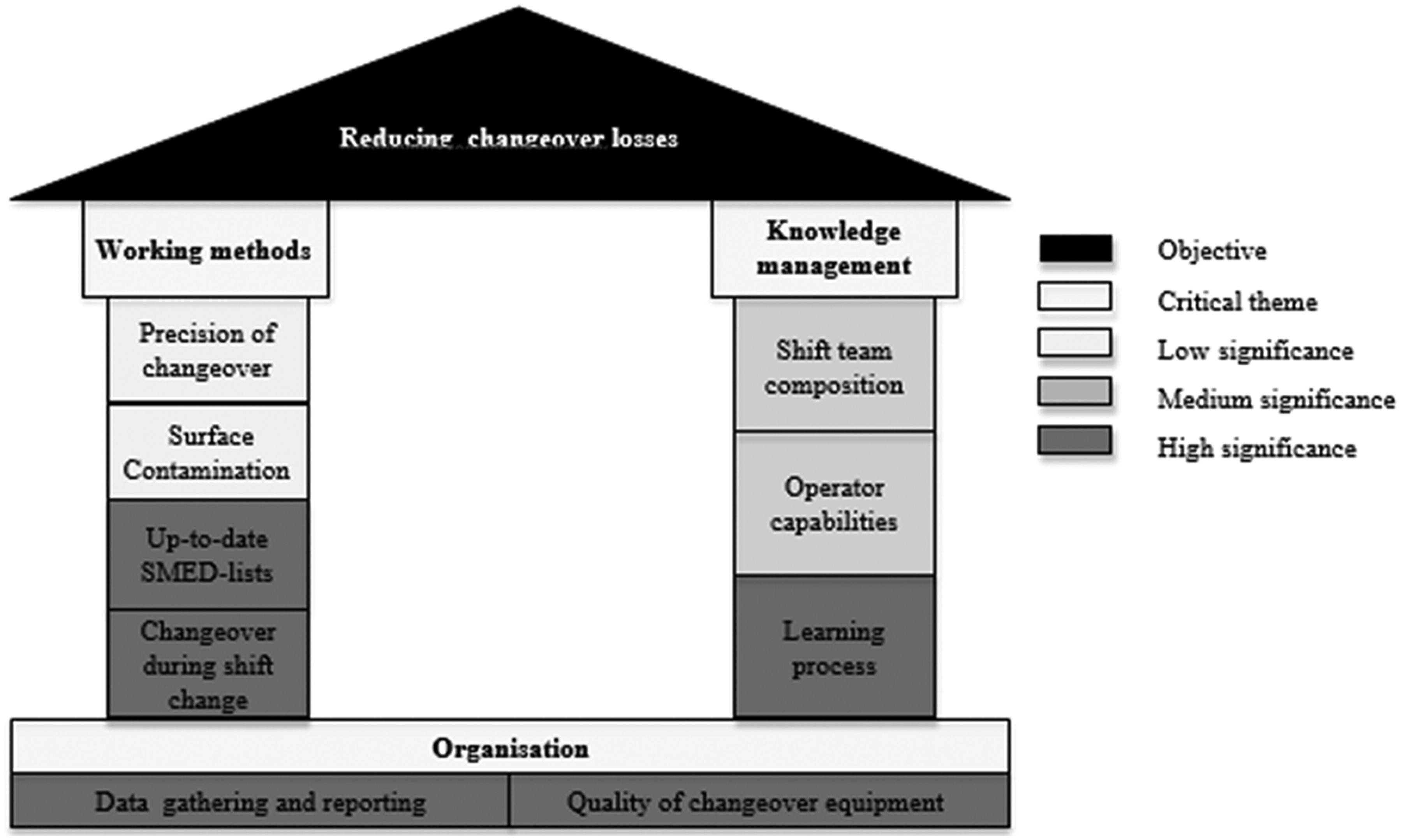
Win the High-Stakes Game of Product Variety With Smart Changeovers
Manufacturing is an ever-changing playing field. With the right CMMS, any plant can build a system changeover strategy that keeps pace with competitors, even when operating with fewer production lines.
MVP One helps maintenance teams of all sizes stay agile without sacrificing quality or compliance. By automating PMs and standardizing changeover procedures, complex processes are documented and streamlined for easy, clear execution.
Whether your plant creates supplements, food, clothing, or any other consumer good, MVP One ensures your equipment is clean and compliant for every production curveball.
Need to supercharge changeovers fast? Schedule a demo or test MVP One on your equipment with a free trial!